Advocacy
Care: equal access to timely, high quality care
Healthcare

As official stakeholders to the World Health Organisation Regional Committee for Europe, EFA contributed to the drafting of the WHO Europe Regional Programme of Work 2020-2025. EFA successfully advocated for the inclusion of measures such as the continuum of care during emergencies, better use of digital tools for self-management, and prevention policies for health and well-being with a special focus on risk factors such as air pollution and tobacco.

One of EU4Health CSA’s aims was to influence the institutional negotiations around the EU4Health budget 2021-2027. EFA and the EU4Health CSA focused joint efforts on advocating for more EU action on health with resourced initiatives.
Together, we published:
- 7 joint statements, including a call for more EU action on health (May).
- 10 guiding principles for the EU health programme (September).
- A statement in view of the European Parliament Plenary vote (November).
EFA also contributed to drafting amendments to ensure ambitious funding for health policies in the next EU budget.
 As Members of the EUnetHTA stakeholder pool, we witnessed the paralysis of the 2018 European Commission proposal for a Health Technology Assessment (HTA) Regulation at the Council of the European Union. Moreover, we experienced difficulties with patient inclusion and representation in the work of EUnetHTA. As a result, we sent a letter requesting the governing bodies adopt a framework for interaction with stakeholders that acknowledged the role of patients, consumers, and healthcare professional organisations. Our efforts underscore the importance and strength of patient organisations in this process; The Regulation will likely be adopted in 2021.
As Members of the EUnetHTA stakeholder pool, we witnessed the paralysis of the 2018 European Commission proposal for a Health Technology Assessment (HTA) Regulation at the Council of the European Union. Moreover, we experienced difficulties with patient inclusion and representation in the work of EUnetHTA. As a result, we sent a letter requesting the governing bodies adopt a framework for interaction with stakeholders that acknowledged the role of patients, consumers, and healthcare professional organisations. Our efforts underscore the importance and strength of patient organisations in this process; The Regulation will likely be adopted in 2021.
Medicines
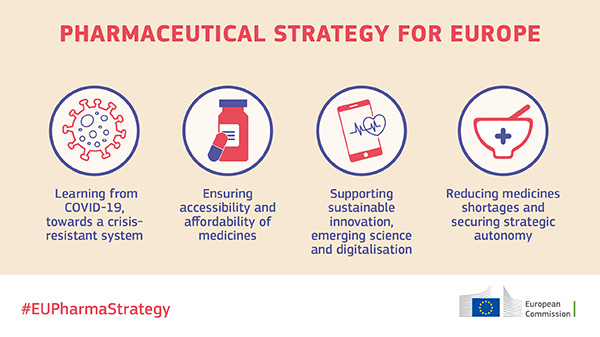
This past year showed that the patient perspective was needed more than ever, particularly in the hastening of medicines and medical devices innovation. EFA participated in the European Commission’s discussions on a Pharmaceutical Strategy for Europe to address key aspects of access to medicines, enable digital health and innovation in general, and draw lessons from the COVID-19 experience to build preparedness ahead of future pandemics.
EFA provided the patient perspective on medicine shortages, the development and authorisation of innovative medicines to treat and prevent allergy, asthma, and chronic obstructive pulmonary disease (COPD), and the rise of digital health.
The European Commission started the process to review the Fluorinated Greenhouse Gases (F-Gases) Regulation to be closer aligned with EU climate objectives. Some F-Gases are a key part of asthma and COPD liquid inhalers that are mainly used as life-saving medication to avoid or stop asthma attacks and COPD exacerbations. EFA communicated our concerns on the F-Gases consultations and called for fully integrated health considerations in all future EU climate policy. While treatment choices can and should be improved to meet environmental requirements, new legislation should not compromise patients’ access to the care they need.
Alongside the review, EFA facilitated a workshop with the European Commission DG Climate Action and other stakeholders including respiratory physicians, hospital representatives, industry associations and a national environment agency to explore different stances on how to reduce F-Gas emissions with minimal impact on patients. The workshop reflected the rising awareness of the importance for health consideration in all policies as well as the strength of patient organisations in facilitating connections across industries.
As members of the European Medicines Agency Patient and Consumer Working Party (EMA PCWP), EFA participated in 3 meetings and 5 stakeholder meetings, including one on COVID-19 vaccines, to represent and bring attention to the issues faced by people with allergy and respiratory disease. Our patient experts were also involved in 1 scientific advisory procedure and the review of 6 different Package Leaflets (PL).
Digital health


In February 2020, the European Commission unveiled the European Strategy for Data, a key driver of the EU digital transition over the next five years. The strategy aims to increase the availability of data and maximise the benefit of data-driven applications by creating common data spaces in areas such as health and the environment.
EFA, as a member of the European Patient’s Forum (EPF) Digital Health Working Group, contributed to the legislative process to structure the European data ecosystem. We participated in the European Commission consultation on the Data Strategy to deliver the voice of allergy and respiratory patients, along with their concerns and expectations of data in healthcare. We also contributed to EPF’s response to the White Paper on Artificial Intelligence and its role in health care.
From consent and privacy to personalised care, EFA demands that the digitalisation of health systems is centred in patient needs, accessibility, health literacy and empowerment.
EFA published a joint submission with the BBMRI-ERIC (a research infrastructure for biobanks and biomolecular resources) to the consultation on the legislative framework for the governance of common European data spaces in July. Together we argued that a health data space ambition should connect researchers with existing data infrastructures, research networks and patient groups in order to advance healthcare.