European Parliament Elections
The new and the old: past European Parliament achievements and elections polls
European Parliament achievements in public health during 2009-2014
During the last 5 years, 766 Members of the European Parliament (MEP) representing 28 countries have adopted decisions in 25 European Parliament (EP) specialised committees and sub-committees, organised 2,819 meetings and adopted 952 legislative acts. There were many discussions, debates and attempts to reach a consensus to significantly improve the health of EU citizens. Now is time to make the conclusions. We have summarised the main achievements of the EP (in cooperation with other EU institutions) in the public health sector in 2009-2014 in order to see what has been done and what will appear on the next Parliament’s agenda.
1) General public health framework
In 2010, the EU launched a ten-year growth strategy Europe 2020 that included health provisions. Those have been completed by the newly Third Health Programme for the period 2014-2020, aimed to promote health, diminish cross-border health threats, contribute to innovative and sustainable health systems, and to facilitate access to better and safer health care for the EU citizens. Just a couple of months before the elections, the Parliament decided to allocate around 450 million Euros for the promotion of public health in Europe.
In addition, stemming from the high-level meeting of the United Nations General Assembly on non-communicable diseases in 2011, the EU announced 2012 as the European year of Healthy Ageing and Solidarity between Generations and launched the European Innovation Partnership on Active and Health Ageing with the objective of ensuring that the number of additional life expectancy years is healthier.
2) Air pollution
Adopted in 2013, the seventh environment action programme until 2020 provides the framework for all environmental actions carried out in the European Union. Pivoted in the three thematic areas, including health, the EU emphasises for the first time, the need for an overarching EU strategy on indoor air pollution, as well as the necessity to have clean air, in line with the standards proposed by the World Health Organisation.
In 2011, the EP started its analysis on the new EU climate and energy framework for 2030, calling for a 40% reduction in domestic EU greenhouse gas emissions by 2030 compared to 1990 levels. The Commission proposed the package at the beginning of 2014, a couple of months after the proposal for the new air quality package. The discussions on the revision of the National Emission Ceilings Directive, which aims at reducing the emission of certain new air pollutants by Member States, have already started and are currently ongoing.
In new estimates released last month, the World Health Organisation reports that in 2012 around 7 million people died – one in eight of total global deaths – as a result of air pollution exposure. Members of the European Parliament have taken up these concerns and we hope that future policy-makers will continue along this pathway with the long-term objective of reducing air pollution to the limit values identified by WHO and to levels that are not harmful for human health.
3) Medicines, medical devices, clinical trials
The EU legislation on pharmacovigilance (Regulation and Directive), adopted in 2010, reinforced and rationalised the system for monitoring the safety of medicines on the European market. It improved patient safety and public health through better prevention, detection and assessment of adverse reactions to medicines. Safety and control of medicines was further strengthened through adoption of the Directive on falsified medicines in 2011.
In 2012, the European Commission proposed two Regulations (1) and (2) as a revision of the medical device directives, aiming to make both medical devices and in vitro diagnostic medical devices more transparent and innovative, thus ensuring better safety of patients and consumers. Several months before, the Commission proposed a new Clinical Trials Regulation, to replace the Clinical Trials Directive, with the objective of ensuring identical rules for conducting clinical trials in Europe. Both dossiers are still under legislative procedure at the European Parliament and EFA suggests to future MEPs and Commissioners to further review them in order to increase transparency, patients’ safety and involvement in decisions influencing their health.
4) Patients
The Directive on cross-border healthcare (2011) clarified patients’ rights regarding access to cross-border healthcare; made necessary provisions to guarantee the safety, quality and efficiency of cross-border healthcare, as well as encouraged cooperation between Member States on healthcare matters. Since October 2013, the European Union is more integrated in terms of healthcare as a citizen is entitled to go to another Member States to be treated for his/her disease, therefore reducing health inequalities in Europe.
The Regulation on the provision of food information to consumers was adopted in 2011 and it will enforce the rules on mandatory nutrition information and better highlight of allergens, also covering non pre-packed food from mid-December 2014.
5) Tobacco control
One of the most significant achievements regarding tobacco control was reached through the adoption of the new EU Tobacco Products Directive, which introduced stricter measures on the labelling of tobacco products, ingredients, tracking and tracing, e-cigarettes, cross-border distance sales, and herbal products for smoking.
6) Research and innovation
In 2013, the EU adopted Horizon 2020, the research programme for 2014-2020, aimed to support research and innovation in various fields, including health. More than 80 billion Euros are available over 7 years to keep adults active and independent for longer and support the development and sustainability of health and care systems. This research programme has almost doubled its budget compared to the previous Seventh Framework Programme for Research (FP7) -the previous research funding initiative that operated from 2007 until 2013-.
Also in 2013, the Commission proposed the Innovative Medicines Initiative 2 (IMI 2) aiming at developing the next generation vaccines, medicines and treatments, such as new antibiotics. Co-financed by the European Commission under the research programme and the European Federation of Pharmaceutical Industries and Associations (EFPIA), and a proposed budget of almost 3.5 billion Euros, IMI 2 was voted in the responsible parliamentary committee (Industry, Research and Energy), but the discussions will continue during the next term.
7) EFA’s disease areas
In addition to the summarised legislation, the Members of the European Parliament also demonstrated their interest in health areas relevant to EFA’s activities, and called the European Commission to:
- recognise the burden of allergic disease (1) (2);
- prevent and treat chronic respiratory diseases including asthma and COPD (1) (2) (3) (4) (5);
- develop an EU strategy to control ragweed, the cause of very severe allergies (1);
- create real-time information system on allergenic pollen in the European Union (2);
- raise awareness in asthma and its causes in children (1) (2);
- develop indoor air quality legislation (1).
MEPs are the only directly democratically elected EU individuals, representing more than 500 million Europeans, and have contributed in many ways to improve the quality of our lives and our well-being. Nevertheless, many challenges still remain. It is up now for EU citizens and patients to value those achievements and vote consequently in order to push the next European Parliament to rise to these challenges, tackle the most pressing issues, help patients with asthma, allergy and COPD to live uncompromised lives and help prevent these diseases.
The main achievements of the European Commission during the past 5 years are available here. To date, health is way too underestimated, but EFA calls on the future policy-makers to change the trend and put health concerns at the top of the next European political agenda.
Summary of polls: how the EU might look like?
It is definitely important to reflect on the leaving Parliament’s activities but it is also very exciting to know how the new European Parliament will look like in terms of programme and alliances.
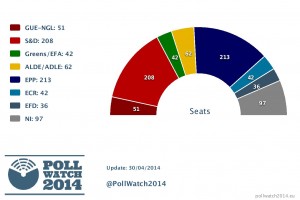
The most recent polls suggest that the European People’s Party (EPP) will lead with 213 MEPs against 208 seats on the Group of the Progressive Alliance of Socialists & Democrats (S&D). However, S&D parties have remained stable in European election polls in most countries, while EPP parties have fallen in France, Finland and Poland.
Alliance of Liberals and Democrats for Europe (ALDE) remains third, just ahead of European United Left - Nordic Green Left (GUE/NGL). The potential fifth place could be shared by European Conservatives and Reformists Group (ECR) and Greens (EFA). Europe of Freedom and Democracy (EFD), a right-wing Eurosceptic political group, remains to be in the last place.
Amongst the non-attached MEPs and not currently attached national parties, it is suggested that the parties from France, the Netherlands, Austria, Italy, Belgium, Sweden and Slovakia could form a new extreme right group.