EU health data regulation enters into force: unlocking access to health records for patients
On 26 March 2025, the European Health Data Space (EHDS) Regulation officially became EU law. This is a major step forward for building a European Health Union that works for patients. The EHDS offers more than just technical solutions. It marks a turning point in how Europeans can access their own health data directly, choose sharing options and improve allergy, asthma and COPD disease management.
The European Federation of Allergy and Airways Diseases Patients’ Associations (EFA) has followed and informed the development of the EU EHDS from the beginning. Since 2020, we have been calling for a data framework that puts patients at the center, protecting their rights and focusing in improving care.
As the EU now moves to implement the EHDS, Member States and stakeholders are designing the complex and robust data systems, rules, and digital infrastructure needed to bring this health data space to life.
A framework to improve care and enable innovation
The EHDS introduces a shared European framework to help health data flow safely between patients and healthcare providers, and meaningfully across borders. It gives people access to their health data and allows them to share it with healthcare professionals throughout the EU. This makes it easier to understand own care pathway and receive care in another country. Importantly, as it is digital and always at hand, it improves continuity between healthcare services and helps clinical information follow the patient.
At the same time, the EHDS will serve to break EU silos, as it regulates how individual patients’ anonymised or pseudonymised data can be used for research, innovation, public health, and policymaking at EU level, helping Europe build smarter, evidence-based systems for the future.
Making digital health work for patients
EFA welcomes the EHDS as an opportunity to re-center healthcare around patients. By improving access to data, this information system can support better self-management and more appropriate care. It also brings Europe closer to a health system that is connected, inclusive and ready for the challenges of chronic diseases.
But to deliver on its promises, implementation must centre on patient rights and trust. People need to be informed clearly about how their data is used and have tangible opportunities to interact and get active on their health. One of the most important objectives in the EHDS is the requirement for countries to improve digital health literacy.
Also, patients are the engine for knowledge development through patient reported outcomes and clinical trial participation, but we welcome the right to opt out from sharing data for research (secondary data use), as well as.
Yet, important gaps remain. The regulation does not provide patients with a clear path to justice if their data rights are violated. And despite the EHDS being built to serve people, patient organisations are not guaranteed a place in its core governance. Without structured representation, key decisions risk being taken without the input of those most affected.
What happens next
The EHDS will be rolled out over the next decade. In 2027, the first detailed rules will be adopted. By 2029, patients should be able to access and share core health data across Member States. More data types, including imaging and hospital reports, will follow by 2031. From 2034, international partners may be able to join the system for secondary data use.
This long timeline reflects the scale of the change ahead. But the direction is clear: a more connected Europe that uses data to support better care, research, and decision-making.
EFA will continue advocating for patients throughout the implementation. We are observers in the European Commission eHealth Stakeholder Group since 2024. We will work to ensure the EHDS is transparent, secure, and inclusive helping it become a tool that delivers not only innovation, but dignity and support for every person living with allergy or respiratory disease.
The EHDS is a chance to make care smarter, fairer, and more accessible. To achieve that, patients must remain at the centre, every step of the way.
Learn more about EFA’s work on EHDS:
EFA response to the public consultation on a European Health Data Space (EHDS)
Refer to the European Commission's FAQ for more info
EFA launches Community support scheme for World Atopic Eczema Day 2025 projects
For the second year, EFA has launched a Community support scheme to support EFA members projects to raise national and local awareness in the lead up to World Atopic Eczema Day 2025.
This support scheme reinforces EFA community impact and will help members raise awareness of the burdens of living with atopic eczema and call for improved access to care.
EFA is more than a federation. We are a dynamic patient community and the funding scheme provides an opportunity to connect the Atopic Eczema community and the broader public, healthcare providers, and policymakers to ensure the voices of those affected are heard and acted upon.
EFA’s support scheme 2025
Through EFA’s scheme, the selected member organisations will receive up to 1,000 EUR to support their communication and dissemination efforts, to improving the prevention and care environment for atopic eczema patients and caregivers across Europe.
Members will propose the activities that fit best their organisation and operational context, from organising local awareness raising campaign, to developing communications materials and implementing media outreach campaigns.
For the 8th consecutive year, EFA is partnering with GlobalSkin in the preparation of the theme for World Atopic Eczema Day 2025, which will be Our skin, our journey (#AtopicEczemaJourney). A communication toolkit and further information on how to join the campaign will be shared in May.
We look forward to working with our community to further increase awareness and call for action about Atopic Eczema this year.
An impactful community support scheme 2024: #AtopicEczemaUnfiltered
Last year, six member organisations implemented impactful activities thanks to EFA’s support scheme.
Through online campaigns, educational workshops, direct consultations, events, distribution of informative materials and many other activities, they reached collectively over 77,000 people on social media, and allowed over 100 patients to receive personalised consultations.
Find out more about the activities organised on World Atopic Eczema Day 2024 in the EFA Community Report.
If you want to learn more about how to become an EFA member and join the community click here.
EFA Community convenes at the 2025 Annual General Meeting and Community Meeting in Slovenia
From 24 to 26 March, EFA Community gathered in Ljubljana, Slovenia, hosted by our member Društvo Atopijski Dermatitis, for EFA’s Annual General Meeting (AGM) and Community Meeting. We welcomed 23 member patient organisations from 15 countries across Europe who came together for three enriching days of discussions, experience sharing and peer-to-peer learning.

EFA Community at the AGM 2025 in Ljubljana, Slovenia
EFA AGM 2025
Marcia Podestà, EFA President, chaired the event and welcomed the EFA Community of allergy, atopic eczema, asthma and chronic obstructive pulmonary disease (COPD) patient leaders: “You, our members, are the heart and soul of EFA and the highest governing body of our community. Together, we bring patient voices where they matter most.”

Marcia Podestà and Mojca Gobec
EFA community was also honoured to welcome Mojca Gobec, Head Disease and Injury Prevention Division at the Slovenian Ministry of Health to open our AGM. “As I look around the room, I see representatives from patient associations across Europe, united by a common purpose to advocate for and empower the millions facing the daily challenges of allergy, asthma, and chronic obstructive pulmonary disease. To make progress, patients, policymakers and healthcare professionals, we must work together.”

EFA Members from Slovenia and Mojca Gobec
First of all, the AGM elected the new EFA Board, composed of new and re-elected members:
- EFA President Marcia Podestà (Food Allergy Italia)
- Vice-president Karoly Illy (Longfonds, the Netherlands)
- Board Member Fríða Rún ÞÓRÐARDÓTTIR (Allergy and Asthma association of Iceland),
- Board Member Anna ANDRALOJC (Polish Federation of Asthma allergy and COPD Patients’ Associations)
- Board Member Simone Miles (Allergy UK)
- Fríða Rún ÞÓRÐARDÓTTIR (Allergy and Asthma association of Iceland),
- Anna ANDRALOJC (Polish Federation of Asthma allergy and COPD Patients’ Associations)
- Simone Miles (Allergy UK)
The EFA Community looks forward to work with the new board and thanks the previous Board members who led EFA in the past two years: Armando Ruiz (FENAER, Spain), Céline Demoulin (AFPRAL, France), Christine Strouss (LongFonds, the Netherlands).
This year we were also delighted to welcome three new member organisations to EFA community:
- Luxembourg Allergy Network
- Allergissima from Swtizerland
- Asociación de Afectados por la Dermatitis Atópica (AADA) from Spain
Allergissima is a Swiss association based in Lausanne, and was founded with the aim of informing, challenging, intervening and acting to improve the quality of life of people with allergy or intolerances. The association achieves this objective by organising and participating in conferences, meetings and various events, writing and publishing press articles, books, booklets related to this specific field.
Luxembourg Allergy Network was founded in 2015 with a vision to accomplish a fulfilling everyday life for the many people living with allergies in Luxembourg. The association works towards an inclusive and sustainable society by developing education and supporting initiatives that raise awareness and facilitate the management of allergic diseases.
The Asociación de Afectados por la Dermatitis Atópica is a Spanish association based in Madrid and founded in 2017 by a group of patients with severe Atopic Dermatitis. Their mission is to improve patients’ quality of life by organising different activities to raise raise awareness of AD, de-stigmatizing the condition, educating, informing and being the voice of patients before health policy decision-makers.
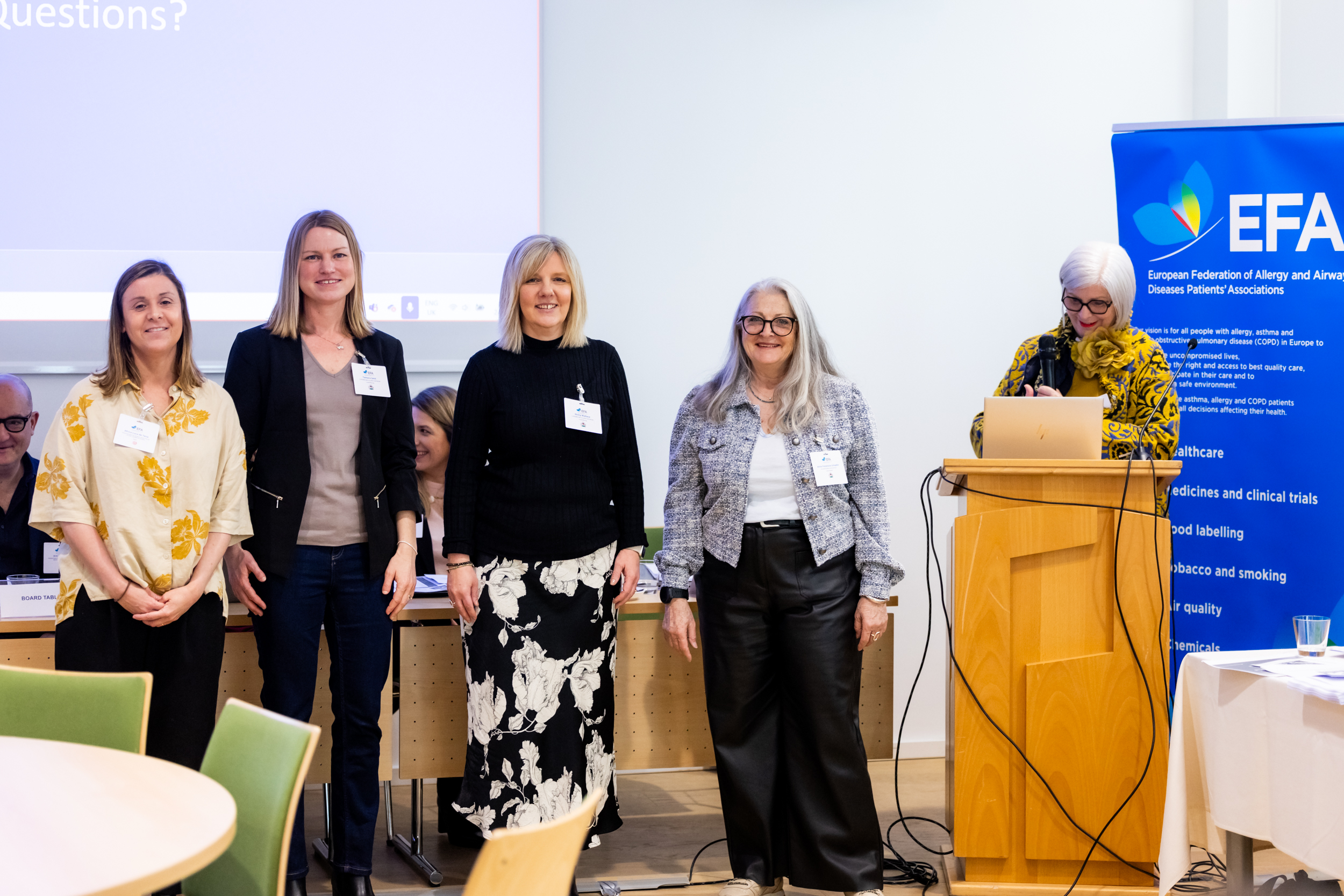
Newly-elected EFA members 2025
Finally, EFA AGM approved the 2024 EFA activity report and accounts as well as the 2025 EFA workplan and budget. The AGM also adopted the first ever EFA Community Policy on Conflict of Interest and Competing Interest that aims at fostering a safe space at EFA and between member associations driven by collaboration, trust and transparency.
EFA Community Meeting 2025
Following the AGM, the EFA members gathered over three days for the Community Meeting under the theme “The Power Within – Growing Together, Sharing Knowledge, Empowering Our Community”. This was an amazing opportunity for members to empower our community bottom up, discuss the advocacy work of the community at large, share insights and best national practices and strengthen the bond between members. Representatives of EFA’s Sustainable Corporate Partners also joined the first two days of the meeting.
During the first day, we discussed how to increase engagement within the Community and how to improve the internal communication and information sharing within the community. We then had the chance to participate in an interactive session who gave insightful tips and ideas on how to improve our respective organisations’ storytelling for more impactful advocacy work.

Day two started with an inspiring session led by EFA members who shared some of their achievements and best practices with the community. It was a great opportunity to learn and be inspired by what fellow organisations are doing. The sessions included:
- “How fit are your lungs”: presentation of a sustained lung function testing activity in shopping centres by the Austrian Lung Union.
- “Nature health”: insights and research studies on our interlinked connection with the environment and how nature can support allergy and asthma health, presented by the Finnish Allergy and Asthma Federation.
- “Writings in pink”: a national literary contest for women living with COPD, presented by the Italian organisation Respiriamo Insieme
- "The Power of Small Victories". Session on embracing everyday wins, finding positivity in challenges, and using our voices to uplift others facing similar struggles led by Špela Novak, founder and leader of Društvo Atopijski Dermatitis

The day continued with an interactive role play involving our members and corporate partners which helped participants step into the shoes of patient organisations, healthcare professionals, policy makers and corporate partners to share insights, explore solutions and drive meaningful change together.
The second part of the day included a 1) session on youth engagement, where members and external speakers shared their experiences on how to create a civic space for young patient voices as well as more opportunities for young advocates to come forward, and a 2) capacity sharing session where two members shared their learnings on fundraising (presented by the Austrian Lung Union) and the use of AI to support our daily work (presented by Društvo Atopijski Dermatitis).
Finally, during the third day, EFA working groups on atopic eczema, food allergy, allergy and asthma and COPD, came together to discuss their priorities and how to best work together to advance on their specific advocacy work.
We are looking forward to bringing all these fruitful discussions into action during the next months and thank all speakers, members and corporate partners for their active participation and insightful exchanges during these three intense and energising days.
EFA advocates for patient safety first in the review of the medical device regulation
On 21 March, the European Federation of Allergy and Airways Diseases Patients’ Associations (EFA) submitted its response to the European Commission’s call for evidence on the evaluation of the Medical Device Regulation (MDR). Based on expert written input and a members workshop, EFA gathered evidence to inform about shortcomings of the current medical devices legislation and recommendations to improve the framework while pursuing allergy and chronic respiratory diseases patient’ safety. We thank participants from Bosnia and Herzegovina, Italy, the Netherlands, and the United Kingdom for their contributions.
Why the EU MDR evaluation matters
The MDR impacts many devices allergy, asthma and chronic obstructive pulmonary disease (COPD) patients rely on – such as spirometers, oxygen concentrators and ventilators – as well as combination products such as inhalers, nasal sprays and injectors. While the MDR, adopted in 2017, has strengthened patient safety, its implementation has posed challenges, potentially affecting access to essential medical devices.
EFA emphasises that any MDR revisions to simplify the regulatory process of development, assessment, authorization and surveillance, must not weaken safety standards. Our recommendations aim to enhance patient protection while improving regulatory clarity and ensuring accessibility.
Key EFA recommendations on medical devices
1. Strengthening patient education. Patients with allergy, asthma and COPD require continuous education on using medical devices correctly and safely, especially if there are combined with medicines. Allergy patients, in particular, need to receive detailed information of allergens present in medical device components, such as latex.
2. Improving inhaler and nasal spray safety. Patients struggle to identify when inhalers are empty or faulty, and these devices do not track total corticosteroid doses, increasing the risk of overdosing. EFA identified that their usability and safety can thus be improved.
3. Increasing patient involvement in medical device regulatory work. To address patient needs, EFA calls for patient inclusion in key MDR bodies such as ethics committees, the Medical Device Coordination Group (MDCG), and expert panels.
4. Enhancing safety reporting. Patients often lack clear pathways to report safety concerns. EFA urges improved accessibility to reporting mechanisms and a stronger role for patient organisations in compiling safety data.
5. Clarifying legislation for combination products. Combined medical products, like inhalers, face fragmented regulatory pathways, leading to risk oversight. EFA recommends recognising them as a distinct category with a dedicated and streamlined regulatory framework.
6. Establishing an EU centralised medical device regulatory dody. Unlike medicines, medical devices lack a centralised EU regulatory agency. A dedicated body could streamline approval processes and ensure equitable device access.
7. Promoting innovation and digitalisation. Technology can improve patient self-management, particularly for those with chronic diseases. MDR should support innovation while maintaining strict safety standards.
8. Ensuring access to spare parts. Chronic disease patients rely on devices long-term, yet spare parts are often unavailable, forcing full replacements. Regulations should facilitate access to non-critical spare parts to reduce costs and waste.
Next Steps
EFA will continue to monitor the MDR review and advocate for patient safety. The full EFA response is available here. Stay connected for further updates on our advocacy efforts.
EU proposal for a Critical Medicines Act to address shortages – EFA perspective
On 27 February, the European Federation of Allergy and Airways Diseases Patients’ Associations (EFA) submitted its input to the European Commission’s call for evidence on the Critical Medicines Act, aligning with the European Patients’ Forum (EPF) response. The Commission published the first proposal for the Act on 11 March, marking an important step in addressing medicine shortages across the EU.
EU’s legislation to regulate availability of critical medicines
Medicines shortages pose a serious public health risk across Europe, affecting access to treatment and patient safety and wellbeing. Due to the complexity and cross-border nature of medicines, a coordinated EU approach is crucial to tackling shortages in a coordinated manner and proportionately across Member States.
The European Commission proposal for a Critical Medicines Act Regulation (CMAR) is an EU priority. It is also a key element of the EU’s broader strategy for an EU #HealthUnion that focus now on securing medicines supply, complementing other initiatives like the revision of the pharmaceutical legislation, the EMA Union Critical Medicines List, and the Health Preparedness and Response Authority (DG HERA) Critical Medicines Alliance (CMA). EFA has been a vocal participating in all these processes and is a member of the CMA.
EFA’s response to the call for evidence on the Critical Medicines Act
For allergy, atopic eczema, asthma, and COPD patients, medicine shortages can be life-threatening and a source of reduced quality of life and economic burden.
EFA welcomes the European Commission efforts to prioritise access in the proposed CMAR but urges legislators to take a broader approach to medicines accessibility that extends beyond supply chain security to fully recognise the patients’ needs and the role of patient organisations addressing medicines shortages.
The following are our key advocacy points:
1. Refine the scope of the act
The CMAR prioritises the medicines listed on the Union Critical Medicines List elaborated by the EMA based on ongoing shortages and risks to health. The latest version of the EMA list includes five respiratory treatments and one for anaphylaxis. However, clarity is needed on which other medicines of common interest could fall within the CMAR’s scope to ensure EU action focuses on the most urgent needs for patients, and also on their preferences and alternative treatments.
2. Expand CMAR beyond industry to fully address public health
The CMAR largely concentrates on strengthening supply chains for medicines considered critical, focusing on the role of industry to ensure supply and the function of national authorities to ensure sustainability through procurement plans and stockpiling. It however fails to consider broader public health concerns, like health system adaptability and the vital role of patients and patient organisations. EFA calls for a broader strategy that includes:
- Use the opportunity of the CMAR to embed patient health literacy on medicine shortages to reduce their impact, through targeted and general awareness campaigns.
- Using patient-reported outcomes to better understand and address the real-world impact of shortages.
3. Ensuring patient involvement in public procurement
Harmonising public procurement rules at the EU level can help strengthen medicine supply chains. However, since shortages impact the delivery of healthcare and the standards of care according to clinical guidelines, EFA calls on the EU to consider long-term impacts on patient outcomes and medicine accessibility. Therefore EFA suggests that patient organisations and healthcare professionals should have a role in procurement and stockpiling discussions at EU and national levels to ensure patient needs are met.
4. Addressing risks in medicine importation
The CMAR promotes collaborative procurement and international partnerships to enhance availability. While this is important, imported medicines must meet EU safety and quality standards, be reimbursed where needed, and be accompanied by clear patient guidance respecting the regulatory requirements for a package leaflet in the local language. Additionally, we emphasise that imports should not create shortages in exporting regions.
Next Steps
EFA’s preliminary analysis of the Critical Medicines Act proposal indicates a strong focus on supply security but insufficient recognition of patient involvement. There are no provisions related to increasing patient health literacy and awareness on shortages.
The Act proposal includes the following chapters:
- Chapter I on general provisions describes the objective, scope and definitions. Next to critical medicines of the Union list, certain chapters also apply to “medicinal products of common interest”. While a definition is given and provides flexibility to Member States to decide when a medicine is insufficiently available and accessible to its patients, it remains too vague and irrespective of the patient need.
- Chapter II on strengthening the Union’s security of supply, which states the strategic objective of the Union and its added value to support Member States.
- Chapter III on enabling conditions for investment introduces the concept of strategic projects on the development of critical medicines, which would enjoy accelerated access to funding and fast-tracked procedures. To be considered a strategic project, it should comply with only 1 out of five criteria described in the Act. It is crucial these projects are accompanied by contractual binding commitments to improve medicine availability. In that sense, impact assessments must be regularly conducted. However, these aspects are not part of the provision.
- Chapter IV on demand side measures obliges Member State authorities to apply other procurement requirements next to price evaluation, and encourages without obligation to look at environmental sustainability and social rights linked to medicines. While this is a step towards harmonizing public procurement in the EU, procurement should also take into account long-term impact on patients, by including patients and healthcare providers in the procurement process. The chapter further obliges Member States to avoid negative impacts of contingency stocks on other Member States as well as allowing for collaborative procurements via different tools.
- Chapter V established the Critical Medicines Coordination Group which facilitates coordination in the implementation of the Regulation. The group, containing Member State experts, the Commission and the Agency as observer, failing to include civil society representatives such as patients organisations.
- Chapter VI on International Cooperation obliges the Commission to explore strategic partnerships to diversify the supply chain of critical medicines. It does not address any of the importation risks related to EU standards, patient impact and solidarity.
- Chapter VII amends the Regulation on Strategic Technologies for Europe Platform (STEP).
- Chapter VIII describes final provisions, including an obligation to evaluate effectiveness and impact five years after its application and every five years thereafter. It does not explicitly state the views of patients and patient organizations should be included.
Moving forward, EFA will continue to monitor the proposal, contribute to public consultations, and advocate for patient-centered policies that ensure medicines remain accessible and safe.
The full EFA response to the call for evidence can be found here.
You can find EPF’s statement on the proposal for the critical medicines act here.
EFA mobilises for the adoption of World Resolution on Skin Diseases
Together with patient organisations around the world and our members across Europe, EFA is mobilising our atopic eczema and skin allergy communities to call on member states to adopt the draft resolution ‘Skin Disease as a Global Public Health Priority’.
The next World Health Assembly (WHA), the decision-making body of the World health Organisation (WHO) will convene from 19 to 27 of May 2025 in Geneva, Switzerland, and will vote, among others, on the draft resolution ‘Skin Disease as a Global Public Health Priority’.
Why is the resolution important?
The draft resolution sets a universal call for better recognition of skin diseases. It covers a wide range of conditions, including atopic eczema and skin allergies and also other diseases such as autoimmune disorders, infectious diseases, rare skin conditions and climate/environment-induced disorders.
Crucially, the draft resolution includes concrete proposals on skin diseases, such as the development of a Global Action Plan for public health response to skin diseases, and supporting Member States to invest in capacity-building, digital technology, prevention measures, diagnosis and surveillance of skin diseases, among others.
What does the resolution mean for European patients?
The draft resolution has been championed by the International Alliance of Dermatology Patient Organisations (GlobalSkin) of which EFA is a member, and the World Skin Health Coalition, of which EFA is partner.
If adopted, the resolution can significantly increase the visibility of skin diseases, pave the way to their integration into national and/or regional health programmes, enhance patient access to treatments and therapies, and help reduce existing stigma.
The resolution is aligned with EFA longstanding recommendations and call to action to address the burden of atopic eczema and to support better patients with severe atopic eczema in Europe.
What can patient organisations do to have #SkinDiseasesResolution approved?
Patient organisations representing people with atopic eczema and skin allergies can contact their Ministry of Health before the 15th of May (WHA is from 19-27 May in Geneva), with an urge to vote positively with the draft resolution. The contacts are available on the WHO website.
GlobalSkin has prepared template letters to address Ministry of Health in three languages (English, French and Spanish). These letters can be tailored to a national situation and include patients testimonials.
They can also be published on the patients’ organisations website and social media channels thanks to template graphic materials to raise awareness among patients networks.
At the EU level, EFA sent an open letter to the EU Permanent Delegation to the World Health Assembly to call for their support of the draft resolution.
38 experts join forces to improve lung health for youth: the #LungHealth4Life Community of Practice kicks off
On 25 February 2025, EFA hosted the first online meeting of the LungHealth4Life Community of Practice (CoP). The event marked the beginning of a collaborative effort to improve lung health for young people and children across Europe, bringing together diverse stakeholders committed to creating a healthier future.
LungHealth4Life project is aimed at laying the groundwork for healthier generations by ensuring all young people have the tools they need to prevent lung diseases. Within the project, the LungHealth4Life CoP is a dynamic, multidisciplinary platform coordinated by EFA, where experts across healthcare, education, and policy join forces to tackle lung health challenges. It provides a space where stakeholders can share knowledge and best practices, helping to create useful tools for educators and healthcare providers, shape resources, influence policies, and build capacity for better lung health.
The first meeting welcomed 38 participants who engaged in productive discussions. They highlighted the challenges health organisations face, such as knowledge gaps, limited access to health promotion initiatives for young children in educational settings and inadequate healthcare training on lung health.
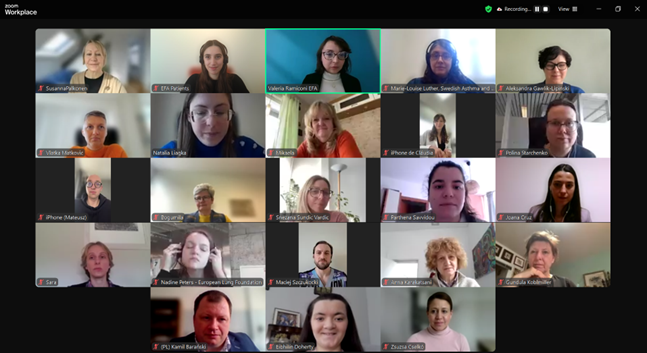
#LH4L CoP group picture
The CoP plays a central role in refining educational materials, advocating for early intervention, and supporting policies that promote better care for children and young people. Members of the CoP have access to exclusive materials, engage in capacity-building activities and are part of an ongoing dialogue that shapes the future of lung health. This collaborative network will ultimately support health promotion and strengthen efforts to reduce the impact of chronic respiratory diseases across Europe.
If you are interested in joining this important initiative, we invite you to be a part of the LungHealth4Life Community of Practice. Join our community here!
This project is co-funded under the EU4Health Programme 2021–2027 under grant agreement no. 101101187. Views and opinions expressed are those of the author(s) and do not necessarily reflect those of the European Union or the European Health and Digital Executive Agency. Neither the European Union nor the granting authority can be held responsible for them.
European Commission prioritises competitiveness, as prevention retreats – EFA perspective on the 2025 Work Programme
On 11 February, the European Commission presented its annual Work Programme for 2025, setting the legislative agenda for the year ahead. This is the first Work Programme under the new Commission, shaped by the political landscape following the last EU elections in June 2024.
As expected, the 2025 agenda reflects shifting political priorities. The focus is clear: EU competitiveness, regulatory market simplification, defence and migration. These priorities align with broader geopolitical and economic challenges, but they also signal a retreat from prevention-focused health policies, which were a cornerstone of the first Von der Leyen Commission’s agenda priorities, namely the EU Green Deal, the European Health Union and Europe’s Beating Cancer Plan.
Public health policy: is prevention no longer a priority for the EU?
For the EFA patient community, the 2025 Work Programme presents a mixed picture. Some initiatives that have started to take shape this year could lead to long-awaited progress for the EU and its neighboring countries in the region:
- The Critical Medicines Act and the EU Stockpiling Strategy. This would be new EU laws aiming to strengthen Europe’s ability to respond to medicine shortages and should help ensure patient access to essential treatments, by incentivising and securing production and supply of critical medicines for the EU Member States. EFA is member of the European Commission Critical Medicines Alliance informing this fiture law, since its launch in 2024.
- The Evaluation of the EU Framework on Medical Devices and In vitro Medical Devices. This exercise will help in assessing what has been the uptake of the new regulation that entered into force in 2021 with the aim of improving patient safety. If the regulation is set to review, it can be an opportunity to address regulatory gaps in medical technologies, especially in drug-device combinations,
- The continued negotiation on the EU Pharmaceutical Package. While this process started in 2020, the current negotiations are decisive on the final shape of the regulations, and we hope it will improve access to medicines and enhance innovation towards patients' unmet needs.
The upcoming months will be crucial in defining patients’ access to treatments and addressing critical gaps in Europe’s health systems. However, the Commission marks a clear shift away from public health and disease prevention as a political priority, in contrast to the previous mandate. Tackling the access, social and environmental determinants of disease is far less prominent in the new plan as actions addressing chronic diseases are absent from the Commission’s agenda, including specific work on tobacco and vaping, indoor air quality and food labelling policies.
A kick-off year for the EU budget for health
2025 marks the start of negotiations for the next Multi-Annual Financial Framework (MFF) for the years 2027-2034. With the annual EU health budgets already declining, Europe must ensure that the lessons from COVID-19 are not forgotten, maintaining a strong commitment to reinforcing health policies, particularly in prevention.
EFA will work with its communities to call on the EU to strengthen the healthcare system with a focus on preparedness, while prioritising public health measures for allergy and respiratory patients, and ensure that their needs are addressed in the EU future policy decisions.
You can find the EC Work Programme and its Annexes here.
EFA Community illustrates why EU Member States should implement the new Air Quality Directive
For most people, breathing is effortless. Yet for millions of Europeans with allergy, asthma, chronic obstructive pulmonary disease (COPD) and severe allergy, it is a daily challenge. Air pollution is not just an environmental issue but an ongoing health crisis. Despite progress in EU legislation, the reality is clear: only the full implementation of the new EU Air Quality Directive can ensure that health risks for patients are reduced.
The EU’s new Air Quality Directive was approved by the European Parliament plenary in April 2024 and by the Council in October 2024. It entered into force in December 2024 and will start applying at the end of 2026. However, there is a real risk that EU Member States may fail to fully implement the measures they agreed upon, as many have done in the past. If this happens, millions will remain exposed to harmful air pollution, leading to worsening symptoms, more emergency room visits, and increased strain on healthcare systems.
At the EU Healthy Air Coalition’s Policy Outlook event, EFA board member Christine Strous made it clear why implementation cannot be delayed. She shared powerful patient testimonies that revealed the human cost of air pollution. Their experiences emphasised the urgent need for stronger national actions to turn EU policy into real protection for those most affected.
The reality of living with air pollution
For many patients, air pollution is an unavoidable daily struggle. One patient shared: "Breathing polluted air is like feeling every breath as a weight. It reminds me every day that my health also depends on the quality of the air." Others spoke of the isolation they experience when pollution levels rise, forcing them to stay indoors. One caregiver described the fear of watching her child struggle to breathe when air pollution peaks, knowing that no inhaler can fully protect them.
For some, the effects are immediate and frightening. Another patient explained: "When air pollution is high, I first feel it in my nose and throat and then I realise that breathing gets harder. It is not normal to feel breathing." One patient even explained how pollution forces them to avoid certain regions in Europe altogether, as poor air quality increases their risk of pneumonia.
Beyond health, the impact extends to mental well-being and social life. "I never really sleep. I check on my child every night, as I did when they were a newborn. They are never again happy to be outside, careless, free. Never!" said one caregiver. Others described how pollution creates economic burdens, with one patient explaining how they are forced to invest in air sensors, protective masks and other costly precautions just to feel safe.
A path forward for cleaner air
The stakes are clear. Member States must fully implement the agreed measures to protect public health. Policymakers need to prioritise those most vulnerable to air pollution and address the real-life impact of air pollution by enforcing stricter pollution limits, improving air quality monitoring and ensuring national action plans are in place. Legislative commitments must translate into concrete action to guarantee that every European citizen has the right to #KeepBreathing cleaner air.
Why indoor air quality must be a European priority
We spend 90% of our time indoors, yet indoor air quality (IAQ) remains overlooked in EU policymaking. For millions of people with allergy and respiratory diseases like asthma, this neglect has real consequences. Breathing should not be a health risk, but without an EU-wide IAQ framework, it often is.

This is why the Mind the Gap: Toward a Comprehensive European Union Act on Indoor Air Quality webinar, co-organised by the SynAir-G and EDIAQI projects, was a timely and urgent discussion. Both projects are part of IDEAL Cluster, a group of seven projects aiming to enhance understanding on the health impacts of IAQ.
As part of the SynAir-G project, EFA talked about the learnings of working on the policy aspects of the Cluster. Importantly, EFA also brought the patient perspective to the table, emphasising the critical need to protect those most affected by poor IAQ.
The science-policy disconnect
EFA’s Panagiotis Chaslaridis addressed one of the biggest challenges: the widespread perception that IAQ is a national rather than an EU-level issue. This assumption has stalled the development of strong, harmonised regulations, despite decades of evidence linking poor IAQ to significant health burdens.
“The EU Treaties give the EU a strong responsibility to protect public health. Given the mounting evidence on IAQ’s health impacts, it’s time for IAQ to be formally recognised as a public health issue that requires an EU-wide response,” Panagiotis stressed.
Another challenge is the disconnect between existing EU regulations and IAQ standards developed by the European Committee for Standardisation (CEN). While scientific advances continue to refine how we measure and mitigate indoor pollution, these insights struggle to find their way into actionable policies.
Prioritising patients
For allergy and asthma patients, poor IAQ is not just an inconvenience, it is a daily battle. Some people cannot even open a window without triggering severe symptoms. Others must always keep emergency medication always within reach, never knowing when an indoor space will become hazardous to their health.
That is why vulnerable groups must not only be considered in IAQ policymaking but actively involved in shaping it. As both Brady and Chaslaridis highlighted, listening to the vulnerable communities and integrating their lived experiences into policy design is crucial.
Public awareness is also key. Despite mounting evidence of IAQ’s health risks, the issue remains largely invisible in public discourse. Initiatives like the EU Green Deal and Renovation Wave Strategy offer potential entry points for IAQ measures, but without public demand and political will, change will remain slow.
Time for an EU-Wide law on indoor air quality
The webinar made one thing clear: IAQ cannot remain a policy afterthought. Europe needs a comprehensive IAQ framework with:
- A common EU definition of IAQ
- A sound legal basis for EU action
- Cohesion with relevant policies
- Minimum standards to protect the most vulnerable
- Strong public engagement to drive awareness and action
EFA and its community of patients will continue to push for better indoor air quality policies through SynAir-G project and beyond. Our priorities remain clear: raising awareness, ensuring strong and harmonised regulations, and advocating for policies that put patients first. We will keep working to make clean indoor air a fundamental part of public health policy in Europe.
Shaping the future of COPD care in Europe: a call for urgent action at the European Parliament
The European Federation of Allergy and Airways Diseases Patients' Associations (EFA) recently brought together at the European Parliament patient advocates, healthcare leaders and policymakers to tackle one of Europe’s most pressing challenges in European healthcare systems: chronic obstructive pulmonary disease (COPD), a preventable chronic disease. The event was co-hosted by MEPs Romana Jerković (S&D/Croatia), Tilly Metz (Greens/Luxembourg) and András Kulja (EPP/Hungary) - all committed to lung health and members of the MEP Lung Health Group.
With over 36 million people living with COPD in Europe, the disease not only impacts health but also strains the social and economic potential of our societies. EFA, in collaboration with our community of members, has published the science-based report “Raising the standards of care for COPD in Europe” calling for urgent action to improve COPD care across Europe. The report offers policy recommendations to improve early detection, access to care, reduce stigma and boost research to improve the quality of life for millions.

COPD is a leading cause of death in Europe, yet it remains underdiagnosed and undertreated. It affects not only the health of people but also their social lives, job opportunities and family dynamics. Many live with breathlessness, making everyday tasks difficult, while caregivers often face overwhelming challenges without adequate support.
This is why EFA brought these issues to the European Parliament, urging for immediate action. As EFA President Marcia Podestà stated in her opening remarks, “COPD is not just statistics, it is people’s lives. EU health policy must reflect that.” Her words were echoed by patient advocates and policymakers, emphasising the urgent need for comprehensive policy changes at European level.
EFA President Marcia Podestà
Breaking the stigma and addressing policy gaps
MEP Romana Jerković stressed the importance of EU political action: "COPD patients cannot afford another decade of inaction. We need EU policies that prioritise early detection, better care, and prevention."
EFA President Marcia Podestà, MEP Romana Jerković, Vice President Armando Ruiz
The stigma surrounding COPD is another significant barrier that hampers progress. Often perceived as a self-inflicted disease, the truth is that COPD affects people from all walks of life. As MEP Tilly Metz pointed out, "COPD is more than a health issue. It is a policy priority. We must break the stigma, strengthen care and ensure no patient is left behind."
EFA President Marcia Podestà, Moderator Sarah-Taïssir Bencharif, MEP Romana Jerković, MEP Tilly Metz
This stigma has a direct impact on policy priorities: in the case of COPD, stigma translates into neglection, delayed diagnosis, fewer resources allocated for treatment and a lack of public awareness about the seriousness of the disease, which is irreversible. Our report calls for a comprehensive approach to changing the narrative, with a focus on prevention and early diagnosis to reduce the burden of COPD across Europe.
The role of early diagnosis of COPD in saving lives
One of the report’s key recommendations is the integration of early COPD detection into healthcare systems. Early diagnosis can dramatically improve the outcomes for patients, enabling them to start treatment before the disease progresses too far. MEP András Kulja highlighted the importance of this in his remarks: "Timely detection of COPD can change lives. We must integrate early screening into national healthcare systems." The evidence is clear: early diagnosis leads to better management and quality of life for patients and saves lives.
MEP András Kulja
Putting faces to the numbers: COPD patients and caregivers
Throughout the event, the EFA community shared powerful personal stories about the life impact of COPD. Paula Duarte from Respira, a leading patient advocate, shared the powerful message, "We must especially advance in prevention so that in the next 20-30 years, we can say that COPD is no longer a pandemic as it is now. " Her words served as a reminder that behind every statistic, there is a patient, a family and a community that is directly affected by COPD.
Paula Duarte from Respira, EFA Member from Portugal
Svetlana Atanasova from Eurocarers stressed that COPD does not just affect patients: "COPD impacts families. Supporting informal carers is key to resilient healthcare systems". Informal caregivers often face emotional and physical exhaustion without sufficient recognition or support on how much their care sustains healthcare systems. This is why EFA is calling for policies that provide informal caregivers with the resources they need to support their loved ones.
Svetlana Atanasova, Eurocarers
Prevention: the foundation for a healthier future
The EFA community is also advocating for stronger prevention policies to reduce the burden of COPD. As Marianne Takki, Acting Head of Unit, Directorate General for Health and Food Safety, European Commission explained, “With 70% of COPD cases preventable, the EU is stepping up on prevention, investing in health literacy, and strengthening tobacco control to reduce the burden of lung disease.” Prevention remains the cornerstone of a healthier future and EFA’s vision for Europe is one where COPD is effectively prevented and no longer a leading cause of death.
The path forward: a call to action to raise standards of care for COPD
With the release of this report, EFA has set a clear path forward, but the time for action is now. The patient-driven policy recommendations outlined in the report are grounded in the lived experiences of those affected by COPD and they offer a roadmap for European policymakers to take concrete steps towards improving care for millions of Europeans. Early diagnosis, access to care and a focus on prevention must become the standard, not the exception.
EFA is committed to advocating for the rights of COPD patients and their families and will continue working alongside healthcare professionals, policymakers and our community of patient advocates and caregivers to ensure that no one is left behind in the fight to raise the bar for COPD care in Europe.
Read the full event report here.
Watch the event on EFA's YouTube channel.
EFA is thankful to its sustainable corporate partners Roche and Sanofi and Regeneron Alliance for their unrestricted grants 2023 for the COPD Standards of Care project that made this Report possible. EFA is thankful to its sustainable corporate partners AstraZeneca, Roche and Sanofi and Regeneron Alliance for their unrestricted grants 2024 for this COPD Beyond Care project.
EFA welcomes EMA plan to draft guidelines on nasal spray treatments
EFA responded to the European Medicines Agency's (EMA) consultation on a concept note to develop new guidelines aimed at ensuring therapeutic equivalence between nasal medicinal products. These guidelines have the potential to make a significant impact on the accessibility and safety of treatments administered through the nose, as nasal sprays are becoming clear options to facilitate medicine intake more easy and faster.
Why are the guidelines important?
Nasal sprays medicines are fundamental treatments for patients living with conditions like allergic rhinitis. Now, there are also innovative nasal products enlarging patient choices, for example in emergency treatments and vaccines. Only in 2024, the EMA approved epinephrine nasal sprays for anaphylaxis (Eurneffy) and influenza vaccine nasal sprays (Fluenz). However, as nasal products differ a lot from other type of products, particularly orally inhaled products (OIPs), EMA has taken the step to develop a guideline specific to the risk-benefits of nasal treatments.
By establishing clear parameters for therapeutic equivalence, these guidelines could pave the way for a wider range of nasal medicines, giving patients more treatment options that are just as effective as existing products.
A path to easier and safer treatments
Nasal sprays clearly offer a less invasive treatment option compared to other methods. Patient groups like children and the elderly often struggle with devices that require a technique (inhalers) or are intrusive (injections), so the availability of treatment options as nasal sprays can make a big difference in their treatment experience. Additionally, nasal sprays could improve patient adherence to therapy, as they generally carry fewer risks and are easier to use.
EFA's call to action
In our response to the consultation, EFA emphasised the critical need to keep patients at the center of the process as more nasal sprays enter the market. We stressed that the EMA must ensure patients are well-informed, enabling them to make the best decisions for their health together with healthcare professionals. Patient education and clear communication are essential to helping individuals transition between treatments without disruption to their care.
A new era for patient access to care in Europe: the EU HTA Regulation enters into force
On January 12, 2025, the Health Technology Assessment Regulation (HTAR) officially came into force, marking a significant milestone in European healthcare, and therefore for patients with allergy and chronic respiratory diseases.
For the first time, the EU has established a cooperative framework to harmonise and streamline the processes that precede the market entry of new health technologies (i.e. medicines, vaccines, medical devices, and other innovative products).
After two decades of intricate technical and policy groundwork, the EU Joint HTA framework promises to make these highly regulated procedures more efficient, scientifically robust and patient-centred.
Why is an EU joint HTA framework needed?
Until now, the scope of the commercialisation of new health technologies — such as medicines and medical devices — has been determined through Health Technology Assessments (HTAs) conducted individually by EU member states. These national assessments evaluate the added value, clinical effectiveness, costs, and broader societal impact of healthcare interventions. However, the fragmented approach has often led to unnecessary duplication, inefficiencies, and inconsistent patient access to same technologies across countries.
What will the EU HTA Regulation change?
The EU HTA Regulation introduces a unified framework to address these challenges by:
- Harmonising processes: It reduces discrepancies in how HTAs are conducted across member states.
- Improving efficiency: By pooling resources at the EU level, the regulation reduces duplication of efforts and accelerates assessments.
- Enhancing scientific rigor: Shared expertise strengthens the quality of assessments.
- Focusing on patient needs: Patients and clinicians will be systematically consulted during HTA preparation, ensuring their perspectives shape the outcomes.
- Engaging stakeholders: HTA stakeholders—including patient organisations—will play an active role in the process.
The regulation introduces joint clinical assessments at the EU level, which will inform the decision-making of national pricing and reimbursement authorities. This harmonised approach will allow new health technologies to reach patients more efficiently and we also hope that it will lead to more equitable access across the EU.
Why does HTA matters to patients?
The HTAR marks a significant advancement for patients. By streamlining HTA processes, it empowers individual health Ministries with EU-level assessments to inform national decisions on which medicines and medical devices should be prescribed, under what conditions, pricing and reimbursement schemes.
Key benefits for patients include:
- Faster access: Harmonised assessments will enable quicker decision-making, reducing delays in the availability of new treatments.
- Wider availability: Patients across all member states will have more consistent access to health technologies.
- Patient involvement: For the first time, patients and their organisations are formally included in the HTA process, ensuring that their real-world experiences and needs are central to decision-making. Having such EU-level patient perspective will be crucial to ensure patients will get the access to the health solutions they need.
What’s next?
The HTAR has begun its phased implementation:
- As of January 2025, it applies to marketing authorisation applications for oncology medicines and advanced therapy medicinal products (ATMPs).
- By January 2028, the regulation will extend to orphan medicines.
- By 2030, it will encompass all medicines, including those for allergy, atopic eczema and airways diseases.
This phased approach ensures a smooth transition while allowing for the gradual scaling of the system. Organisations like EFA will remain actively involved, monitoring the regulation’s implementation and contributing to the HTA Stakeholder Network to represent patient interests.
Protecting students with food allergies: EFA and GA2LEN publish joint action plan on anaphylaxis management in schools
For children and youth with food allergies, the classroom is not just a place for learning, but also a space where careful precautions are essential to be healthy. Even a simple snack or lunch break can pose significant risks, creating challenges for students, their families and school staff.
EFA has collaborated with the Global Allergy and Asthma European Network (GA2LEN) to address this critical issue for children and young patients. The result of this collaboration is the launch of the joint statement ‘Towards a common approach for managing food allergy and serious allergic reactions (anaphylaxis) at school’, which was published on 29 December 2024 in Clinical Translational Allergy, one of the leading scientific journals worldwide.
Building on the momentum of the publication on 29 November 2024, this joint action plan focuses on creating safe, inclusive and prepared educational environments, from childcare to universities.
A European food allergy community action plan
The joint action plan is a truly European food allergy community result. On the patient side, it has been led by EFA President Marcia Podestà (Italy) as the first author, and it has been co-authored by EFA member representatives Céline Demoulin (France), Mikaela Odemyr (Sweden), Ángel Sánchez Sanz (Spain) and Sabine Schnadt (Germany). The publication provides a broad perspective of the European need to manage food allergies in educational settings.
Why schools must be ready for anaphylaxis?
Students spend about one-third of their time in educational settings, relying on teachers, canteen workers and other staff to protect them from exposure to food allergens. When anaphylaxis strikes, symptoms can escalate quickly, requiring immediate action to prevent life-threatening outcomes.
At a time of increasing prevalence of food allergy, as well as anaphylactic reaction incidents, it is important to ensure that countries and regions adopt the right and safe protocols for managing serious allergic reactions in schools.
Four practical steps for safer schools
The joint GA2LEN/EFA consensus statement outlines four practical steps to improve food allergy and anaphylaxis management in schools:
- Empower school staff through training about food allergy, its symptoms and psycho-social-emotional aspects, as well as the use of emergency medication. Training led by experts, including patient organisations can improve awareness.
- Focus on prevention through internal policies that prioritise clear food labelling in both prepacked and non-prepacked foods, proper cleaning of eating areas and addressing stigma related to students with food allergies.
- Prepare school establishments for emergencies, including the availability and storage of adrenaline devices, and clear processes in case of a student requiring emergency care.
- Build an inclusive school culture and wider awareness, that informs all school population about food allergy, while aiming to reduce isolation and bullying.
Bridging the gap between policy and practice
Many countries have policies addressing these aspects, but inconsistent implementation undermines their effectiveness, leaving patients unsecured and even unprotected. Educational settings and their workers can learn from best practices where comprehensive approaches have succeeded, demonstrating the life-saving potential of coordinated action.
EFA is grateful to our community of members involved in this invaluable and empowering scientific publication for the whole Food Allergy patient community.
You can access the full paper here.
The EU expands the list of critical medicines: what is in it for allergy, atopic eczema, asthma and COPD care?
On the 16th of December, the European Medicines Agency (EMA) published the updated version of the critical medicines list (CML), marking a significant step in securing the supply of essential medicines in the EU, to treat allergy and respiratory diseases among others.
The list identifies medicines deemed critical based on two criteria: those whose insufficient supply could result in serious harm to patients and who have also been on shortage. The list now includes 288 medicines, an increase of 28 compared to the first version. Among all the critical medicines, the list includes five key respiratory treatments, addressing conditions such as asthma and COPD.
What are the critical allergy and respiratory medicines for the EU?
The following five respiratory medicines, previously listed in the first version of the list, remain critical for patients with airways diseases:
- Salbutamol: A bronchodilator that opens the lungs and is used for asthma and COPD (short-acting β2 adrenergic receptor agonist). The inhalation products with salbutamol are in shortage in most EU/EEA countries and shortages persist due to increased usage and manufacturing issues.
- Ephedrine: An essential for acute bronchospasm (alfa and beta adrenergic receptor agonist). No current shortages reported.
- Ipratropium: An inhaled bronchodilator for asthma and COPD (anticholinergic medication). While specific products face shortages (e.g., albuterol sulfate and ipratropium bromide inhalation solutions by Cipla), alternatives remain available.
- Methylprednisolone: A corticosteroid for allergic rhinitis and asthma. Shortages are noted for methylprednisolone sodium succinate powder for injection but other options are available.
- Acetylcysteine: An expectorant aiding the elimination of lung secretions, for example in episodes of acute pneumonia and bronchitis. Intravenous formulations face no shortages, although some oral and inhalation solutions are limited, but many other products available.
- Epinephrine: cardiac stimulant used for anaphylaxis due to severe allergic reactions (anaphylaxis) resulting from food allergens, insect bites, respiratory allergens and other allergens. There are remaining and frequent shortages for epinephrine autoinjectors but other autoinjector remain available.
- In addition to these critical medicines, the list includes antibiotics, antifungals and vaccines crucial for combating respiratory diseases like pneumonia, influenza, diphtheria and severe fungal infections like aspergillosis.
Medicines for allergy and dermatology not considered critical
As representative of patients with allergy, atopic eczema and airways diseases, EFA actively participated in the stakeholder consultation for the review of the Critical Medicines List in May 2024. However, the EU Member States and Norway decided not to include our suggestions to the list, like the addition of antihistamines as front like treatment against allergic reactions.
One of the reasons not to include them provided by the EMA is that the market supply for EFA disease areas such as allergy and atopic dermatitis is rather good and not facing major EU cross-border disruptions.
Future areas for improvement
EFA welcomes the second version of the Critical Medicines List as a tool to safeguard the supply of essential medicines in the EU. We are encouraged that our response raised awareness among Member States and call on them to promptly report shortages at the national level to the EMA.
We remain a source of information to Member States and the EMA, looking forward to advance on two points that have not been included in the methodology of the list yet.
Seasonal reflection: shortages often worsen in winter, when bronchodilators are in higher demand, prescribed to patients infected with respiratory viruses. EFA calls for seasonality to be considered by EU and Member States when stockpiling for critical allergy and respiratory medicines.
Focus on children: children face a higher risk of medicine shortages due to limited age-appropriate formulations. Greater attention to pediatric needs can reduce reliance on off-label use of medicines among children, and enhance safety.
Looking ahead, EFA will monitor the implementation of the Critical Medicines Act, including joint procurement and stockpiling. These measures are expected to take shape during the next European Commission mandate.
Is the EU better prepared for a pandemic? EFA’s evaluation of the EU serious cross-border threats to health regulation
While COVID-19 was hitting hard the world in March 2020, the European Union started reviewing its legal tools and financial instruments to address health threats more effectively. One of the laws that was reinforced in speedlight was the Regulation (EU) 2022/2371 on serious cross-border health threats (SCBHT regulation).
The SCBHT regulation provides for strengthened coordination and collaboration between the Commission, EU agencies and Member States in the prevention, preparedness and response to a health emergency. The law includes obligations for sharing and reporting, and it sets down decision-making mechanisms. It also aims at establishing and/or strengthening EU level capacities and procedures for surveillance, early detection, risk assessment and rapid response. The Regulation replaced Decision 1082 from 2013.
Is the EU better prepared for a pandemic?
The European Commission launched the first survey to evaluate the functionality of the 2022 SCBHT Regulation. EFA replied to the consultation in December 2024 as it is essential to ensure allergy and lung patients are not left behind in the event of a health emergency.
EFA supports the EU’s legal and financial capacity to respond to health threats across borders in a coordinated manner and with solidarity. Thanks to the SCBHT Regulation, the EU and Member States can better cooperate with each other, since it introduces stress tests on EU assessment of national plans and capacities on cross border health threats.
The role of civil society in emergency preparedness and response needs to be strengthened
In the evaluation, EFA highlighted the gap in the involvement of civil society in health emergency preparedness and response. Despite the regulation not obliging for it, considering the voice and capacity of civil society when Member States prepare their national health emergency plans is essential for success against health threats. Not only participatory mechanisms help increase transparency but they also enhance public trust. Furthermore, one of the learnings of COVID is that better tailored information to the public during health emergencies is crucial for an effective response.
Reducing healthcare shortages is key to preparedness
EFA also pointed out that in a context of continuous health workforce shortages, health emergencies further increase the gap between health needs and healthcare capabilities, which can have fatal consequences for patients. While the regulation alone will not generate more healthcare professionals or reduce existing waiting lists, persistent health workforce shortages should be prioritized given the ageing trends and the likelihood of cross border health threats hitting the Union.
Addressing the existing epidemics: chronic diseases
EFA stressed that the regulation should recognize the already existing burden of non-communicable diseases (NCDs), which are first leading cause of death in the EU. As representatives of patients living with respiratory diseases (1 in 8 deaths in the EU yearly), we urged the Commission and Member States to also address chronic diseases when defining preparedness and response plans.
Climate change is a health crisis
Lastly, while the regulation supports preparing and managing for certain health threats, it does not support health threats due to climate change, despite many of its nature and consequences translating into cross-border impacts, with fatal and hazardous impacts to health.
EFA looks forward to the progression of this evaluation and to the upcoming steps aimed at improving the Regulation.
10 life-saving steps to manage anaphylaxis: a joint manifesto by EFA and GA2LEN ANACare
Food allergy can profoundly impact daily life, turning ordinary moments into potential crises. For some, a simple meal or snack can lead to a life-threatening emergency.
Recognising these challenges, the EFA food allergy patient Community proudly contributed to a key publication for the prevention and management of anaphylaxis, titled as ‘10 practical priorities to prevent and manage serious allergic reactions: GA2LEN ANACare and EFA Anaphylaxis Manifesto’.
Published in early December, the paper united 13 patient organisations worldwide, spanning Europe, Canada, Australia and the US. It was developed in partnership with the Global Allergy and Asthma European Network (GA2LEN) ANACare programme. Among the authors, EFA President Marcia Podestà and member representatives Céline Demoulin (France), Mikaela Odemyr (Sweden), Angel Sánchez Sanz (Spain) and Sabine Schnadt (Germany) made significant contributions.
Doctors and patients united around ten life-saving priorities
This joint initiative is a landmark example of synergy between patients and doctors in the area of food allergy. Written in accessible language, the manifesto is designed for a broad audience, including healthcare professionals from various specialties, policymakers and patients.
The manifesto identifies 10 key patient-centred priorities to prevent and improve immediate and long-term management of anaphylaxis. These priorities address immediate and long-term challenges:
- Increasing awareness on how to identify, respond to and proactively manage severe allergic reactions, including via public information campaigns
- Train existing and future healthcare professionals to proactively support people at risk
- Ensure that anaphylaxis is categorised as a medical condition in all countries
- Implement integrated care pathways across healthcare systems for better access to risk assessment, diagnosis, education and dietetic support
- Educate the food, hospitality and travel industries about food allergy and best communication towards patients
- Empower patients through the provision of clear self-management plans, including when and how to use adrenaline
- Ensure that health professionals are trained to use quality of life assessments
- Improve access to and legal oversight of approved adrenaline devices, including the prescription of two adrenaline devices for people at risk
- Invest in easy access to psychoeducational support to address the burden of people living with allergy and their carers
- Take necessary legislative measures to enable information, including mandatory Precautionary Allergen Labelling (PAL) in prepacked products
EFA is committed to continuing working with the scientists and healthcare professionals’ community to bring forward evidence to improve lives of people with food allergy.
You can access the full manifesto here.
EFA shares asthma patients’ perspective on EAACI Guidelines for indoor air quality
Indoor air can be a silent threat to those living with asthma, filled with pollutants that trigger or worsen their condition.
In November, we worked with our #EFACommunity of asthma members to contribute to the draft Guidelines of the European Academy and Allergy and Clinical Immunology (EAACI). The guidelines aim to bridge the gap between the latest scientific developments and their application for better asthma care, and we thank the medical society for this opportunity.
Science meets real-life challenges
Scientific guidelines are international medical consensus documents developed using a strict scientific methodology. The ones from EAACI therefore reflect the perspective of allergologists on how indoor air quality (IAQ) impacts asthma, including new-onset asthma and disease-related outcomes. They focus on four major pollutants: Volatile Organic Compounds (VOCs), dampness/mould, pesticides, and cleaning/disinfecting products.
While acknowledging that asthma arises from a complex interplay of genetic factors and environmental exposures, the guidelines seek to improve prevention, care and mitigation measures. This is pursued in the shape of recommendations addressed to healthcare professionals, patients, policymakers and citizens, alongside research priorities for the future.
Patients’ input for a solid IAQ framework that improves asthma prevention and care
Recognising the guidelines as a thorough, science-driven reference tool, we welcome its potential to fill critical knowledge gaps on asthma triggers and improve individualised management plans.
Asthma patients live with the reality of polluted indoor air every day. EFA’s review of the draft guidelines brought the the different perspectives of asthma patients’ organisations to the core of the research. EFA highlighted the need to expand the scope of the guidelines by including significant pollutants currently overlooked, including emissions from heating and cooking, house dust mites and tobacco smoke. Everyday products like perfumes, hair care, baby products are also linked with asthma.
Practical measures for real impact
EFA strongly supported EAACI’s recommendation to incorporate assessments of indoor exposure into individual asthma management plans. We emphasise that the critical clinical history factor (indoor air quality as a trigger of their disease or worsening) must be communicated to patients, empowering them to take proactive risk-reduction steps. EFA therefore encouraged EAACI to include clear guidance on implementing avoidance or reduction strategies.
For patients and healthcare professionals, simple, affordable tools to measure, record and monitor indoor air quality are essential. These tools would be especially beneficial in the primary care settings, where initial asthma management often begins.
Focus on ventilation
Ventilation systems, particularly demand-driven ventilation (DCV), play a crucial role in mitigating indoor air risks. However, EFA stressed the importance of evaluating their effectiveness and identifying potential downsides. This issue is particularly urgent in schools, where children spend long hours in closed spaces, sharing the same air. A well-functioning ventilation system could significantly reduce their exposure to asthma triggers.
The draft EAACI guidelines represent a crucial step toward addressing the impact of indoor air pollution on asthma. By prioritising clear, actionable guidance and considering real experiences of patients with asthma, we can create indoor environments that support health for all. EFA office thanks its members Longfonds from the Netherlands, Allergy UK and the Swedish Asthma and Allergy Association for their valuable contributions enriching the EFA input to the draft Guidelines, despite the short timeframe for comments.
We look forward to continue collaborating with EAACI and for the publication of the guidelines.
Shaping the future of food safety: EFA attends the European Food Safety Authority Stakeholder Forum
On 27 November, EFA participated in the seventh Stakeholder Forum meeting of the European Food Safety Authority (EFSA) in Brussels. This year, the meeting focused on a timely and critical theme: the impact of innovation on food safety risk assessment.
Balancing progress and protection
We live in an age of rapid technological advances and evolving food systems. Information technologies, new methods for food risk assessment and novel foods are reshaping the landscape of food safety. At the core of the discussions was a key question: how can we balance the need for efficiency and transparency in risk assessment while ensuring robust regulation?
EFA’s advocacy for safe and inclusive practices
As an accredited stakeholder at EFSA since 2017, EFA participated in the meeting to connect with experts and stay informed about the emerging trends in food safety. While the forum attracted many participants from the food industry, particularly those involved in marketing authorisations, EFA’s focus remained clear – safeguarding consumers from food allergens in both common and novel foods.
We support innovations in risk assessment but insist on a strong regulatory framework to ensure food safety is never compromised. Transparency in risk assessment is not just a technical requirement, it is vital for protecting public health and maintaining consumer trust despite today’s complex food systems.
Addressing the risks of novel foods
Novel foods present exciting opportunities but also unique challenges, especially for patients living with food allergy. Certain proteins found in novel foods, such as insects, are identical to common food allergens such as shrimps. This creates a potential risk of cross-reactivity which is dangerous for food allergy patients.
EFA calls for comprehensive and inclusive allergenicity assessments in novel foods to address these risks. We look forward to collaborating, as a community of patients, with the European Commission as it revisits the EU novel foods framework.
You can find more information about the programme and the discussions here.
EFA responds to EMA guideline consultations on inhalation and nasal medicines for allergy, asthma and COPD
Inhalation and nasal medicinal products are crucial in the treatment and management of allergy, asthma and chronic obstructive pulmonary disease (COPD) and must be of the highest quality. In November, EFA brought the patients’ perspective to two draft scientific guidelines under revision by the European Medicines Agency to reflect optimal pharmaceutical quality and the therapeutical equivalence of new and existing inhalation and nasal products.
EFA praises EMA’s efforts to ensure all inhalation and nasal medicinal products meet the highest quality standards and fully supports that the introduction of new products into the market respect quality and safety standards. We also welcome the increasing interest of pharmaceutical companies to develop technologies that aim to addressing personalised or unmet needs of patients. Innovative products might come with a better safety profile or a less burdensome treatment scheme, resulting in improved adherence to treatment and further enhancing their quality of life.
EFA patients recommendations on the draft guideline on the pharmaceutical quality of inhalation and nasal medicinal products
- Drug-device combination products – The legislation for combination products is currently fragmented in the EU. EFA believes that these products should be recognised as a separate category of medicinal products in the legislation. This would help streamline their development and ensure patient involvement throughout the life cycle of the product, which at the moment does not happen at all or only partially.
- Testing for real-world situations and usage- Test results of quality aspects should be valid in real-world situations and usage of the medicinal product, for example when traveling with the product or when using a spacer. In addition, instructions for safely and correctly handling the products and the robustness of their performance should also be tested throughout the lifecycle of the product.
- All patient profiles are addressed – Patients with different characteristics, diseases and disease severity may have different dose, usage and needs regarding the product. This should be reflected when checking product quality aspects. EFA made multiple comments to make sure all patients in the patient population concerned are represented when the quality of inhalation and nasal medicinal products is tested.
- Avoid potentially inflammatory excipients – The use potentially inflammatory excipients, like lactose, should be avoided as much as possible and, when used, allergen/intolerance and adverse reactions information should be clearly disclosed in the package leaflet.
- F-gases as excipients and research of novel excipients - Fluorinated greenhouse gases (F-gases) are often used in current metered-dose inhalers (MDIs) empowering effective inhalation, but they also impact the environment. EFA recommends novel excipients and propellants to be investigated on their therapeutic equivalent.
- A ml scale on all packages of liquid medicinal products – All manufacturers should be obliged to include an ml scale on the container of all liquid inhalation and nasal medicinal products, especially on opaque glass containers. This way, patients are aware at all times of how much product is left, leading to better self-management of their care and medicine use.
- Identifying last dose for pressurised metered dose inhalation products (pMDIs) – EMA should require that for all pMDIs, the last dose can be recognised by the patient to avoid an empty pMDI is continuously used without the patient knowing, which can have severe health implications.
EFA patients recommendations on the draft guideline on the requirements for showing therapeutical equivalence between new and existing orally inhaled products for asthma and COPD
Similarly to the the guidelines above, EFA highlighted the importance of representing all patient profiles when therapeutic equivalence is demonstrated and the need for real-world testing. In addition, EFA also submitted the following specific recommendations:
- A balance between patient safety and access to medical products – EMA should strike the right balance between patient safety and access to medicines through putting in place strict but relevant requirements for demonstrating therapeutic equivalence between a new/generic orally inhaled medicinal products and an orally inhaled medicinal product already on the market.
- Different product performance of different nebulisers – Since the performance of inhalation products for nebulisation varies greatly depending on the nebuliser used and mixture administered (dual and triple therapies), EFA recommends that all the nebulisers and mixtures mostly used with the reference inhalation product should be tested for therapeutic equivalence.
- Risk of medication error with reformulated dosage – in case the dosage strength of the test product must be changed in comparison to the reference product (e.g. when bioavailability of the test product is higher), it must be clarified by EMA how the potential risk of medication errors must be addressed and in case additional measures are needed, these should be tested with patients and validated.
- Extrapolation of results from adults to children – EFA stresses to be cautious when allowing to extrapolate results from studies on adults to adolescents (>12 years) and children (<12 years). Children are not small adults, so simple extrapolation to the pediatric population should not be allowed.
Next steps
You can find EFA’s full response to consultation on guideline on the requirements for showing therapeautical equivalence between new and existing orally inhaled products for asthma and COPD.
EMA will consider and look into the submitted comments by stakeholders and concerned individuals, before publishing the final guidelines. We hope they will take into account the needs and perspectives of patients with allergies, asthma and COPD as well as of their caregivers.










