Led by the 3TR consortium, a European multi-stakeholder working group has developed ‘Core Outcome Measures sets for paediatric and adult Severe Asthma' (COMSA). Core outcome measures (COM) are a set of tools that should be used in all studies about a specific topic. You can learn more about COM in this short video. The COMSA will serve as a basis for future clinical trials and allow researchers to compare results between studies more easily. They were formed under the leadership of Ekaterina Khaleva, Graham Roberts and Anna Rattu from the University of Southampton, and developed by representatives from across Europe and North America.
Until now, asthma researchers have not used a standard set of COM to understand whether or not biological therapies work. The above-mentioned working group has filled this gap with the COMSA, which will enable better data creation and assessment of biologics in childhood and adult asthma clinical trials. The study was supported by the European Lung Foundation (ELF) and European Federation of Allergy and Airways Diseases Patients' Associations (EFA) to understand the perspectives of people living with severe asthma. With this strong backing, the following COM were selected by the 3TR researchers, patients, clinicians, pharmaceutical representatives, and health authorities.
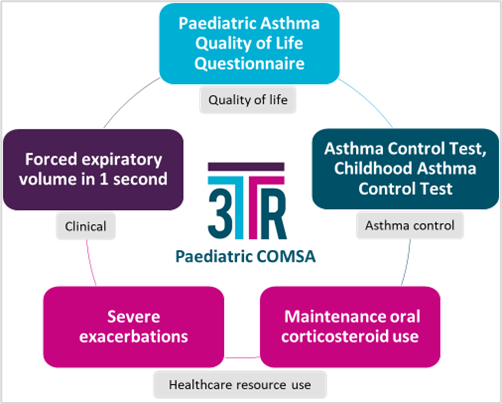
Using the Paediatric and Adult Core Outcome Measures Set (as below) can improve patient’s outcomes when evaluating the success of a biological treatment for severe asthma.
 |
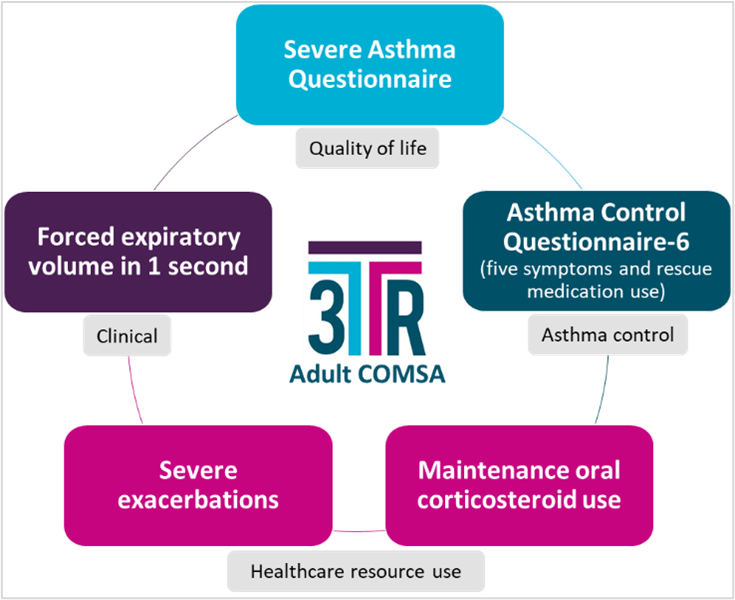 |
3TR's asthma experts expect that the results of this patient-centred collaboration will help inform which COM are used in future clinical trials, improve the ability to compare the effectiveness of biologic medicines, help assess economic value of biologic medicines, optimise therapy for people with severe asthma and improve their quality of life.
Helen Parks, 3TR Respiratory Patient Working Group representative commented on this achievement: "I found the experience to be incredibly insightful and felt that I was contributing to a meaningful activity. The patient voice is key in my mind to ensuring a good outcome for all parties. There is nothing more demoralising than feeling you are ignored as the patient. Contributing in thus process was rewarding, enjoyable and made me feel I could help other patients with severe asthma have a better experience than I have had as a patient. Sometimes how asthma can impact on day to day life is more important to the patient than say a medical aspect. In this was COMSA gets the widest perspective and encourages patient input which is vital.’’
Does your doctors know about the COMSA? Share this new tool with your healthcare team so your care is personalised and considers core outcome measures that we know are important to patients. Are you planning a clinical trial to evaluate the effectiveness of biological treatments for severe asthma? Consider using the COMSA as your core outcome set for more comprehensive and patient centred results, that can be easily compared across studies. Check out the publication of the 68-member group of authors on the COMSA Study.
3TR consortium advocates for a paradigm shift in the way we look at diseases and offers opportunities to shape patient-centred research. The 3TR projects will have significant implications for future patient management and the assessment of efficacy, safety and quality of future health products. Learn more about the project on 3TR website.